A new vaccine for RSV has been included in the upcoming fall vaccine options for seniors, although convincing them to undergo the three-shot regimen is challenging.
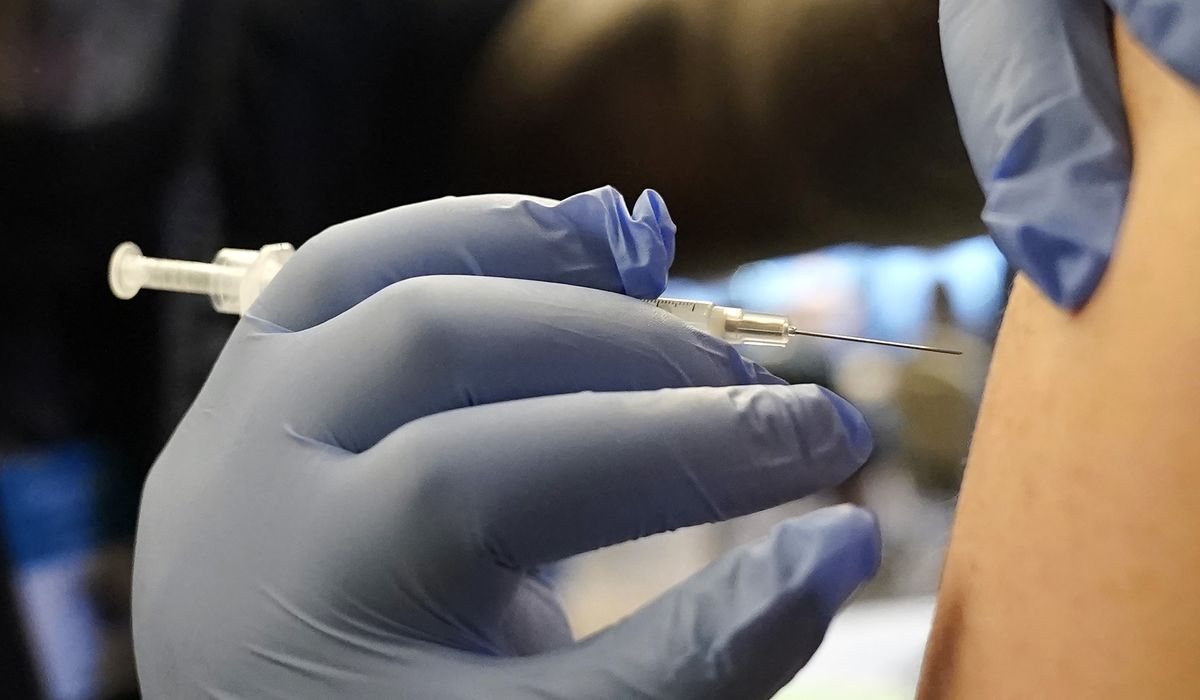
U.S. authorities and pharmaceutical companies are beginning to advertise newly developed vaccines for respiratory syncytial virus. Their aim is to encourage older adults to receive a combination of RSV, flu, and COVID-19 vaccinations in order to maintain clear lungs and minimize the need for hospitalization during the upcoming autumn season.
Doctors urge patients to get a flu shot each year, and Dr. Anthony Fauci and pop star Olivia Rodrigo twisted America’s arms so that pharmacists could poke them with COVID-19 shots during the pandemic.
Drug manufacturer GlaxoSmithKline is currently airing advertisements and recruiting NBA icon Earvin “Magic” Johnson to endorse the initial vaccine for RSV, a virus that frequently affects infants but can be fatal for older individuals, particularly those with pre-existing health issues. Pfizer also obtained approval for its own RSV vaccine a few weeks later, and both vaccines will be introduced to the market this autumn.
Federal officials are recommending the RSV vaccines for persons 60 and older under a “shared clinical decision-making” framework, meaning patients and doctors should discuss the vaccine instead of treating it as an automatic recommendation.
Medical professionals state that older individuals, especially those with heart and respiratory problems, will be ideal candidates for receiving all three vaccinations targeting RSV, influenza, and COVID-19. However, this is contingent upon their willingness to receive the vaccines.
“I anticipate that there will be a considerable amount of individuals who express, ‘Alright, I’ll receive two doses simultaneously, but I’ll return for the third one. That third shot will probably be for RSV, and it will require some convincing to ensure their return for that third appointment,” stated William Schaffner, a specialist in infectious diseases at Vanderbilt University. “This year is going to be a period of learning.”
RSV has been present for a considerable duration, but it had a significant impact in the autumn of 2022, leading to worries about a potential “triple pandemic” in conjunction with the flu and the ongoing coronavirus. The illnesses reached their highest point early, approximately in November. However, pharmaceutical companies shared clinical evidence indicating that their vaccines could effectively prevent RSV in individuals aged 60 and above.
Drug manufacturers and those responsible for immunization are now informing senior care facilities and healthcare providers who cater to elderly individuals.
“The significant prevalence of illness we experienced in the previous year has certainly generated considerable attention. Healthcare providers who understand the impact of RSV-related diseases are pleased to have access to these novel vaccines for their patients aged 60 and above,” stated Litjen Tan, the chief policy and partnerships officer at Immunize.org. This organization is dedicated to advocating for vaccination by providing educational resources to both the general public and healthcare practitioners.
He mentioned that it is difficult to determine the level of public interest, although the challenging season experienced last year made people more aware of RSV.
Dr. Tan acknowledged that while it is possible to administer all three shots simultaneously, it is unlikely that most adult patients would be willing to receive three vaccines at once. Therefore, it may be necessary for the patient to return for subsequent doses.
According to federal data, more than 90% of American seniors received a two-dose primary series for COVID-19, but only around 43% of them chose to receive an updated booster last fall. The Centers for Disease Control and Prevention reports that flu shot coverage among seniors has improved and now stands at over 70% as of March.
Adding a third shot will be a key test of seniors’ willingness to roll up their sleeves this fall. Interest in the COVID-19 shots lagged over time, and the pandemic and associated mandates fueled a new era of vaccine hesitancy in activist circles and corners of the internet.
“I expect a greater challenge in persuading individuals to receive the vaccination,” expressed Claire Hannan, the executive director of the Association of Immunization Managers. “That is precisely why it is crucial to inform healthcare professionals and engage in meaningful conversations with patients regarding the significance of receiving all three vaccines.”
The Food and Drug Administration in May approved the GSK vaccine, which has the brand name Arexvy. The company estimates that nearly 56 million American seniors could benefit from the RSV vaccine.
GSK referred to official statistics indicating that RSV leads to approximately 177,000 hospitalizations and an estimated 14,000 fatalities among elderly individuals in the United States annually. Pfizer markets their version under the brand name ABRYSVO.
The vaccines were given priority-review status, which means that regulatory focus is directed towards drugs and vaccines that would be considered “significant improvements” compared to current pharmaceuticals or treatments.
The CDC endorsed both vaccines in June. Agency spokeswoman Kathleen Conley said doctors and patients should consider whether the patient has any chronic medical conditions or other risk factors that increase their risk of severe RSV disease; possible side effects such as fever or soreness at the injection site; and “the patient’s preference around getting the shot.”
In August, regulators announced that Pfizer’s version of the vaccine could be administered to pregnant women to provide immunity to newborns during the initial six months of their lives. This marks the introduction of the first RSV vaccine designed for maternal use, which is expected to generate increased interest in these new immunizations.
“I cannot reword.”
GSK has initiated a significant television advertisement to endorse its vaccine specifically designed for the elderly. This ad is currently being broadcasted on prominent network channels as well as online platforms such as the Today Show, GMA, Big Bang Theory, and NBC Nightly News.
“RSV is a potentially serious condition. Consult with your doctor or pharmacist to learn more about Arexvy, the first FDA-approved vaccine for this virus. Don’t let RSV control you, choose Arexvy.”
Mr. Johnson, the former L.A. Lakers star, is raising awareness about the disease and vaccine on his social media pages through the “Sideline RSV” campaign. Mr. Johnson previously promoted GSK’s HIV drugs after his battle with the virus.
GSK stated that their RSV vaccines underwent extensive testing to ensure safety and effectiveness. The results showed that the vaccines were 82.6% effective against RSV in older adults during one season and 67.2% effective over the span of two seasons. In terms of severe RSV, the vaccines demonstrated 94% effectiveness for one season and close to 79% effectiveness over two seasons.
The company plans to address vaccine hesitancy that remains from the pandemic era by emphasizing its data and trusted sources.
“The company stated that most Americans have trust in the safety and effectiveness of vaccines. They acknowledged that in recent times, there has been a sense of weariness towards vaccinations due to the pandemic. However, the company remains dedicated to providing accurate information to patients and healthcare professionals, including doctors, pharmacists, nurses, and public health officials.”
Pfizer informed CDC advisers in June that their vaccine demonstrated a 78.6% efficacy against severe RSV during the second season, which was slightly lower than the nearly 90% efficacy observed during the initial season.
Health professionals will utilize the upcoming year to determine whether the vaccines provide sufficient long-term protection, thereby eliminating the need for annual vaccinations.
“I cannot reword”
Pfizer is also a main player, alongside Moderna, in providing COVID-19 vaccines and boosters that match circulating strains.
The companies informed Congress that they intend to increase the list price of the shots by approximately four times, reaching around $130. This decision is due to their entry into the private market and the absence of government support in purchasing.
Pfizer stated that they have set the price of the vaccine in a way that aligns with its value and aims to provide uninterrupted access for all Americans. They anticipate that the majority of individuals will not have to pay anything directly as the COVID-19 vaccine becomes more widely available.
Pfizer announced that it will be prepared to distribute booster shots targeting the XBB lineage by the conclusion of August, subject to regulatory guidance.
Ms. Conley mentioned that the CDC specialists have revised the COVID-19 booster availability to be around mid-to-late September. Additionally, she stated that getting vaccinated for the flu during September and October is typically advisable.

